| |
|
|
| |
| Rubber and History |
- Rubber trees or the scientific name Hevea brasiliensis were brought to Malaysia in 1877 and has continued to be developed as an important commodity in our country until the present day.
- Among Malaysia’s major exports are rubber gloves, condoms and catheters.
- Latex is a white fluid obtained from rubber trees when the tree bark is tapped.
- Natural rubber that is found in latex is a natural polymer known as polyisoprene.
- Latex is a colloid and not a solution.
- Rubber particles do not dissolve in water but disperse separately in water.
- Monomer for polyisoprene is isoprene or with the IUPAC name 2-methylbut-1,3-diene.
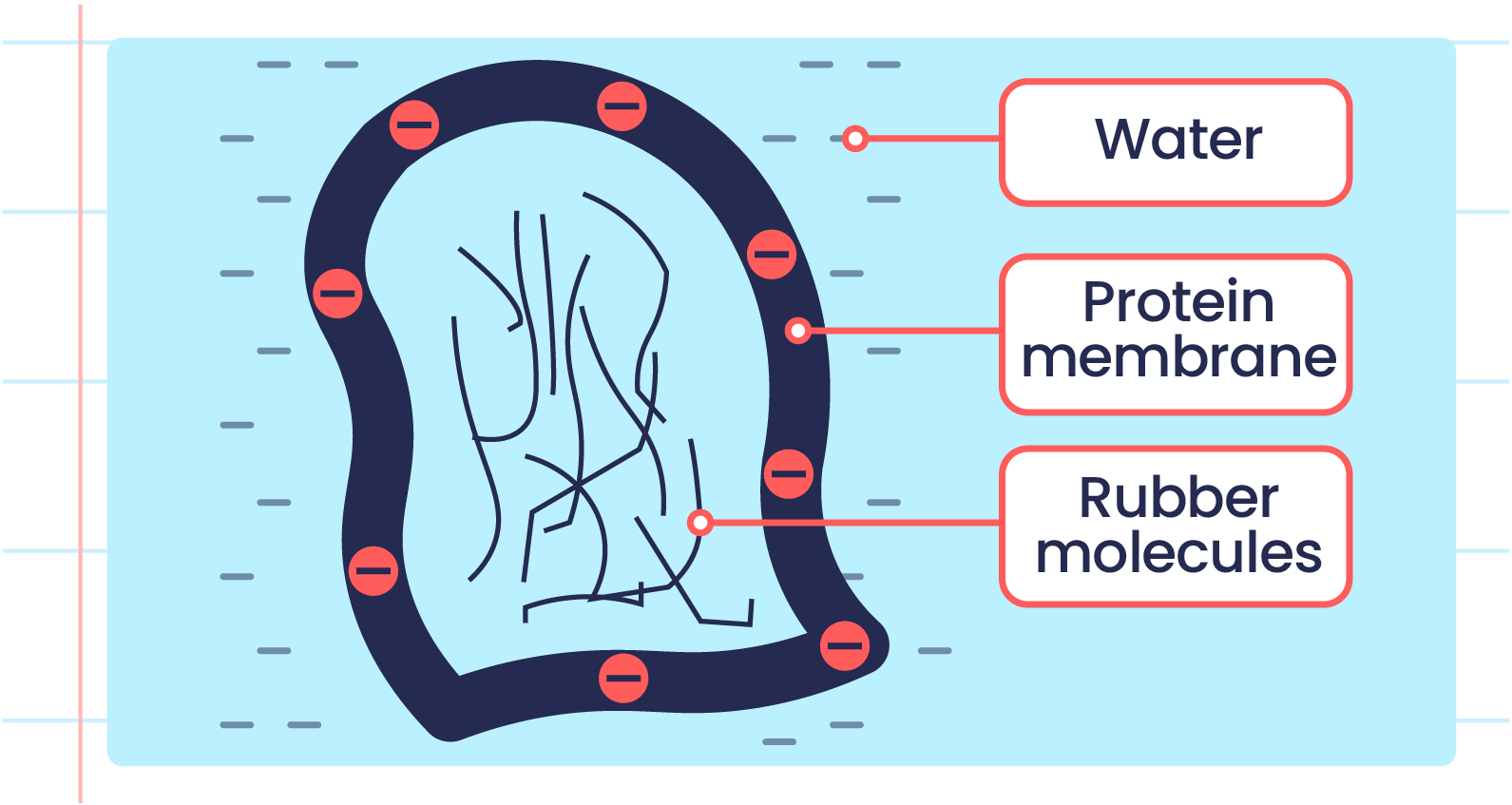
- The protein membrane of rubber particles is negatively charged on the outer surface.
- This causes rubber particles to repel each other, preventing coagulation.
|
|
| |
| Structural Formula of Isoprene or 2-Methylbut-1,3-diene |
|
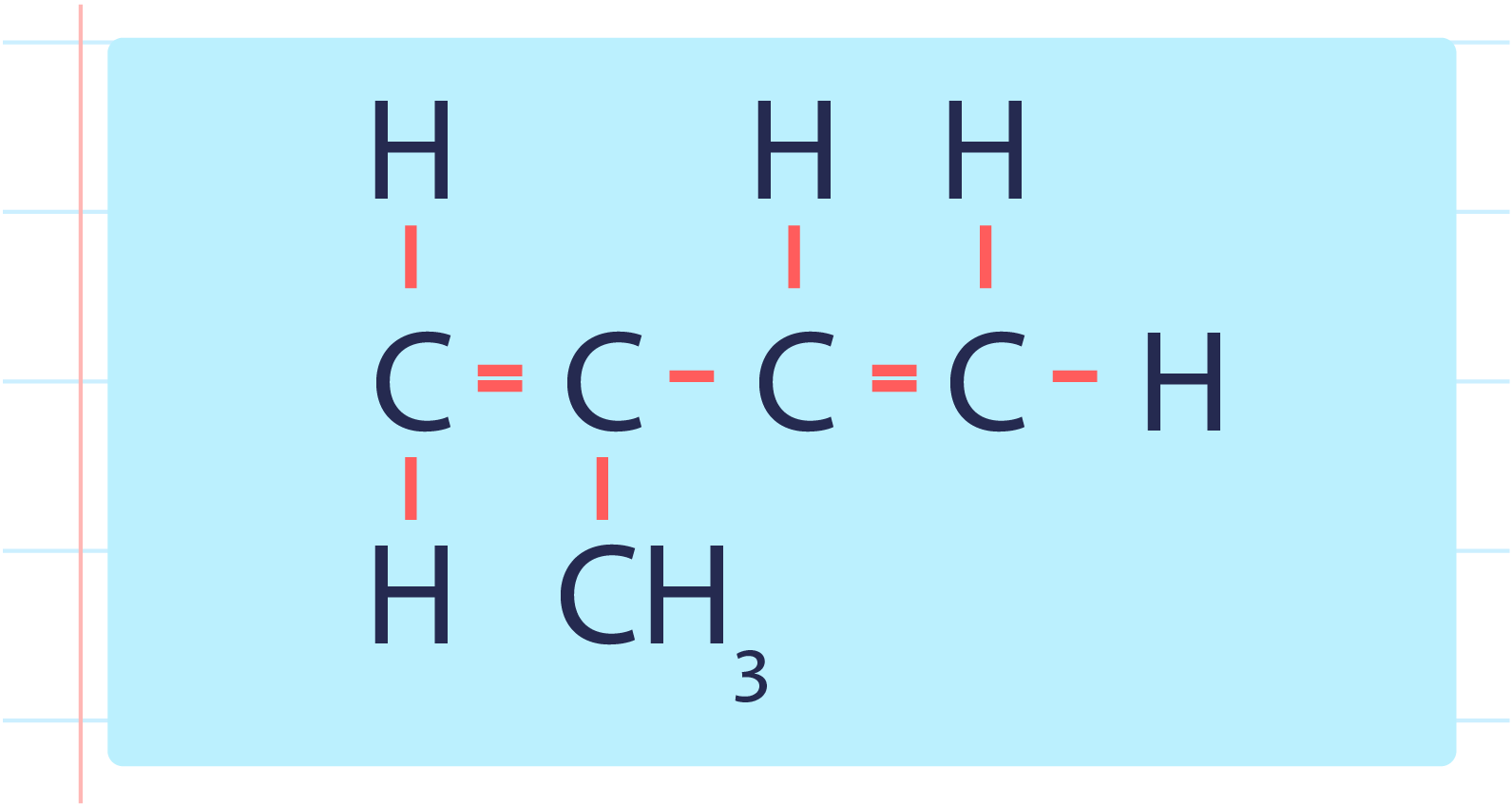
|
|
| |
| Cross Section of Rubber Particle |
 |
|
| |
| Characteristic of Natural Rubber |
- The characteristics of natural rubber depend on rubber polymers that are natural elastomeric polymers.
- The presence of double bond C=C in the rubber polymer structure affects the resistance of natural rubber towards oxidation by air.
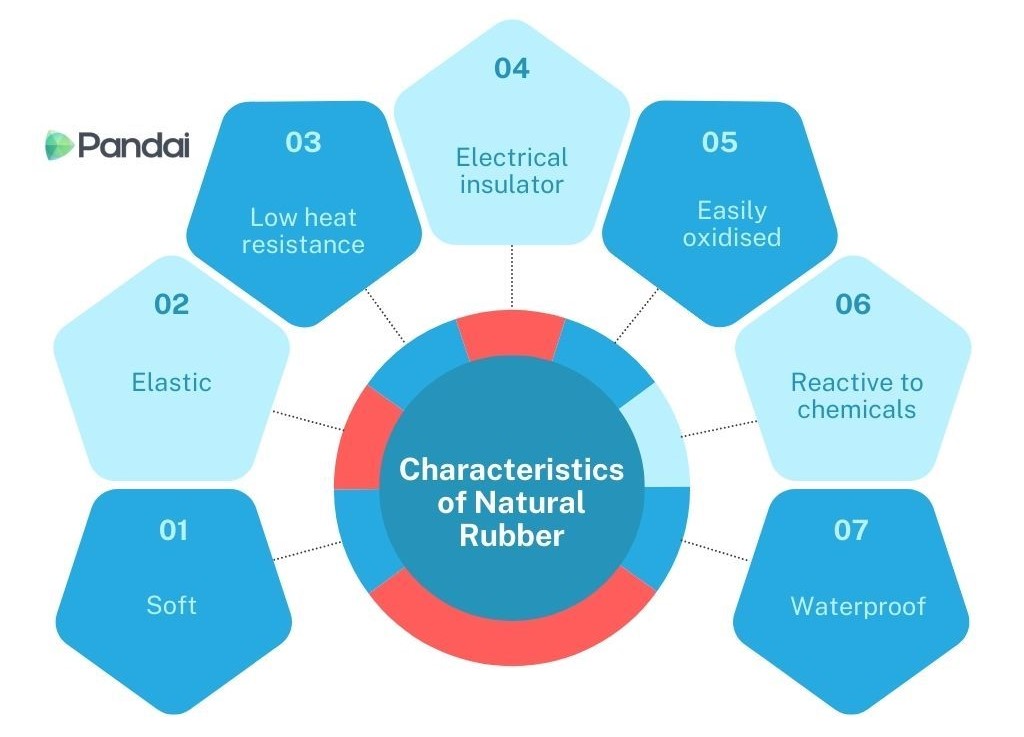
- The characteristic of natural rubber is as shown below:
|
|
| |
 |
| |
| Characteristic of Natural Rubber |
- Soft white solid at room temperature.
|
- Natural rubber can be stretched and can return to its original shape when released.
|
- At high temperature, natural rubber will become soft and sticky.
|
- Natural rubber cannot conduct electricity and is a good electrical insulator.
|
- Oxygen in the air can react with the double bond between carbons and cause natural rubber to be oxidised.
|
- Reacts easily with acids, alkalis and organic solvents.
|
- Natural rubber is water impermeable.
|
|
| |
| Extra Info |
- The Malaysian Rubber Board is an agency responsible for the rubber industry in Malaysia that includes conducting studies on the latest technology involving natural rubber.
|
|
| |
| Products from Natural Rubber |
- Tyre
- Rubber boots
- Rubber band
- Gloves
- Soles of shoes
|
|
| |
| Coagulation of Latex |
- Latex is collected in liquid form for processing or it can be left to coagulate and to be collected a day after it was tapped.
- The collection of latex in liquid form should be done quickly because once exposed to the air for a few hours, the latex will start to coagulate and turn solid.
|
|
| |
| Coagulation Process of Latex |
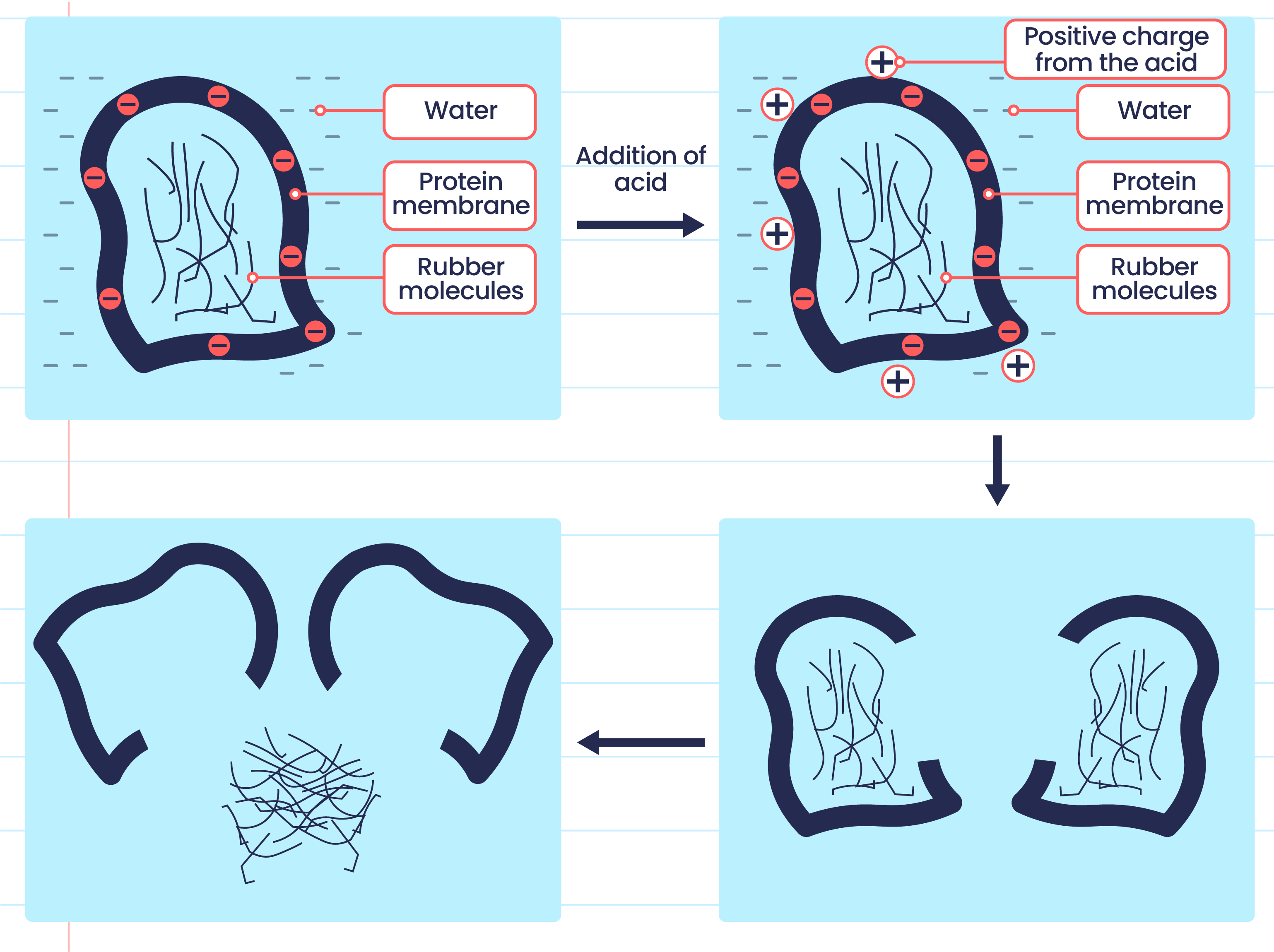
- Acid can coagulate the latex by neutralising the negatively charged protein membrane.
- Coagulation can take place by adding a weak acid into the latex, or it can occur naturally by leaving the latex exposed to the air.
- Bacteria in the air enter the latex and secrete lactic acid, which eventually causes the latex to coagulate.
- The coagulation process of latex is as shown below:
|
|
| |
| Step |
Explaination |
| 1 |
The negatively charged protein membrane causes rubber particles to repel each other. |
| 2 |
Hydrogen ions, \(H^+\) from acid neutralise the negatively charged protein membrane. |
| 3 |
Rubber particles collide with one another that causes the protein membrane to break. |
| 4 |
Rubber polymers combine with one another that causes latex to coagulate. |
|
| |
| Ways to Process Latex Before Taken to Factory |
- Sheet rubber
- Block rubber
- Cup lump rubber
|
|
| |
| Latex Cogulation Prevention |
- Latex is also needed in liquid form to produce specific products, such as gloves and rubber tubes.
- Alkaline solutions such as ammonia, \(NH_3\) is added into the latex to ensure that coagulation does not take place.
- Alkaline solutions consist of hydroxide ions, \(OH^−\) that can neutralise the acid produced by the bacteria.
- The protein membrane of rubber particles remains negatively charged and rubber particles will continue to repel when they draw near one another.
|
|
| |
| Definition of Vulcanisation of Rubber |
| A process of producing rubber that is more elastic and with better quality through the production of cross-links between polymer chains. |
|
| |
| Vulcanisation of Rubber |
- Natural rubber is soft and easily oxidised when exposed to the air for a long period of time.
- This condition renders natural rubber unsuitable to be used for certain conditions or applications.
- The characteristics of natural rubber can be improved by undergoing the vulcanisation process.
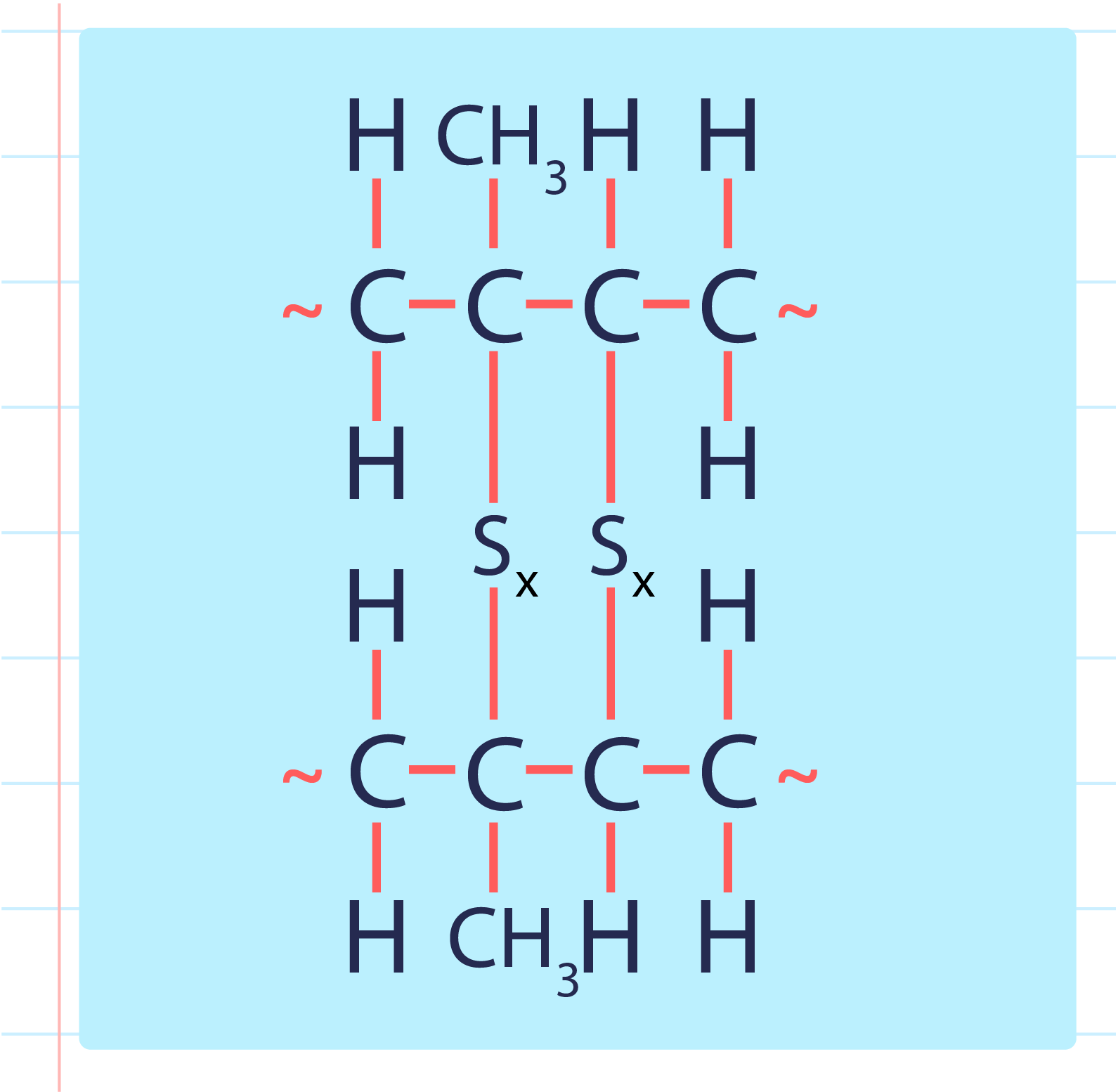
- During a vulcanisation process, the double bond between carbons found in rubber molecules will react with sulphur or other substances to produce sulphur cross-links.
- The vulcanisation of rubber to form sulphur cross-link is as shown below:
|
|
| |
| Vulcanised Rubber |
 |
|
| |
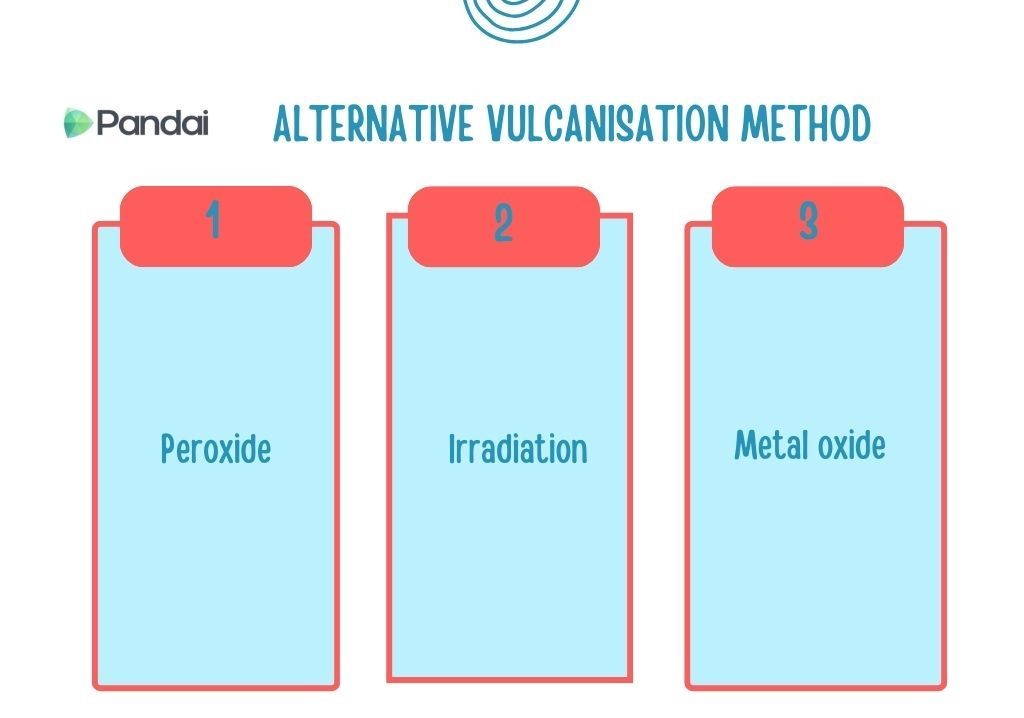
| Alternative Vulcanisation Method |
- Vulcanisation by using sulphur is the main method used to produce vulcanised rubber from natural rubber.
- Nonetheless, this vulcanisation method cannot be used for certain types of rubber, particularly synthetic rubber which does not contain C=C.
- There are a few alternative vulcanisation methods that can be utilised to produce vulcanised rubber without using sulphur.
- Vulcanised rubber produced is free from sulphur and more environmentally friendly.
|
|
| |
 |
| |
| Properties of Vulcanised Rubber |
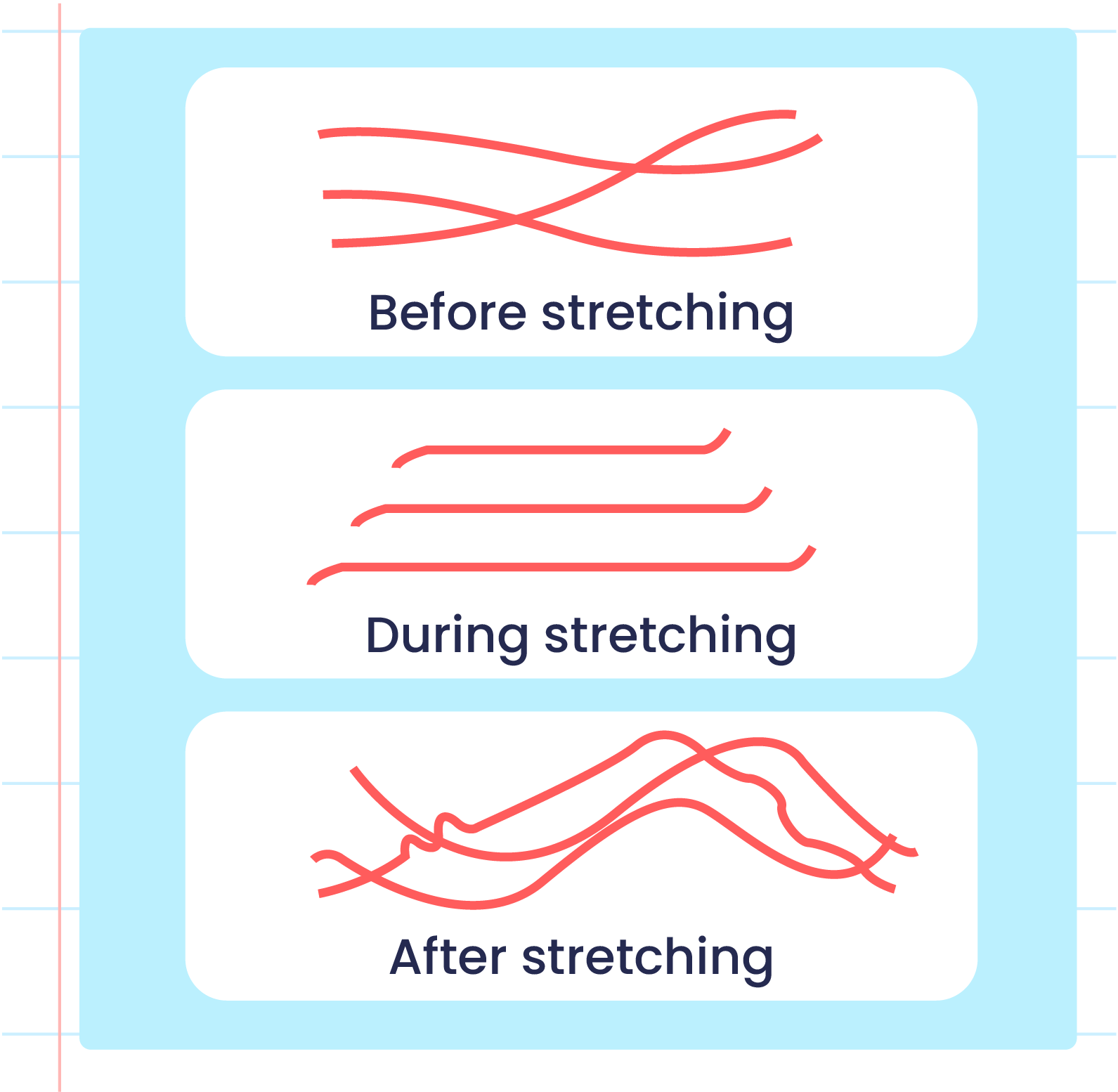
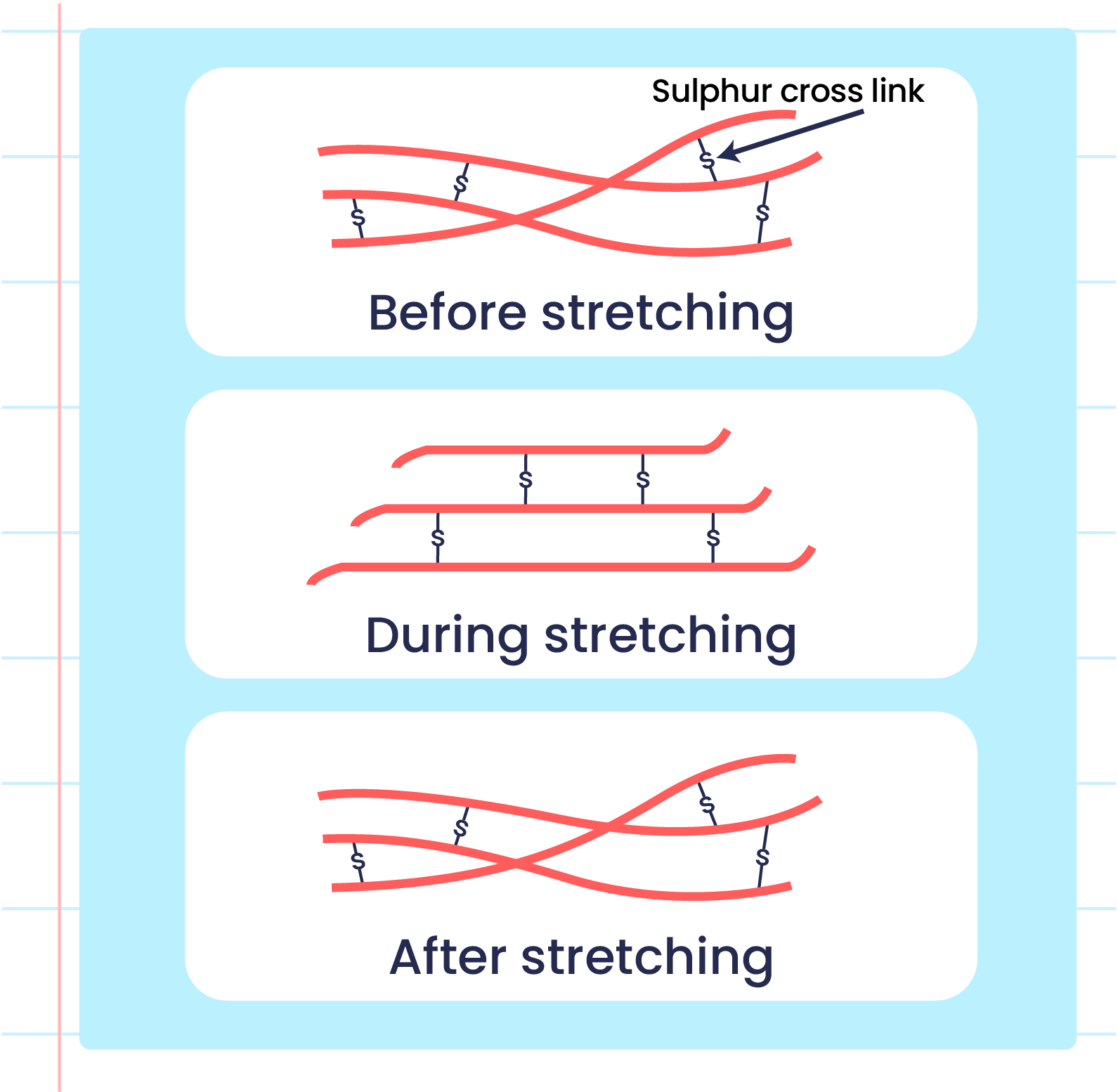
- Vulcanised rubber has different characteristics from unvulcanised rubber due to the formation of cross-links during the vulcanisation process.
- The formation of sulphur cross-links reduces the double bonds between two carbon atoms in vulcanised rubber that makes it harder to be oxidised.
- Strong sulphur cross-links prevents the rubber polymer from sliding when it is stretched and can return to its original shape when released.
- High heat energy is needed to break the linkage.
- This condition makes vulcanised rubber more elastic and has high heat resistance.
- These unique characteristics of vulcanised rubber allow various items to be produced compared to unvulcanised rubber.
|
|
| |
| Illustration of the Elasticityn of Vulcanized Rubber Polymers |
|
|
|
| |
| Illustration of the Elasticityn of Unvulcanized Rubber Polymers |
|
|
|
| |
| The Differences between Vulcanised and Unvulcanised Rubber |
| Characteristics |
Vulcanised Rubber |
Unvulcanised Rubber |
| Elasticity |
More elastic |
Less elastic |
| Hardness |
Hard |
Soft |
| Strength |
High |
Low |
| Resistance towards heat |
Resistant to high heat |
Less resistant to high heat |
| Resistance towards oxidation |
More resistant towards oxidation |
Easier to be oxidised |
|
| |