| SULIT |
2 |
4541/1 |
| |
|
|
| 1. |
|
Manakah antara berikut bukan kenyataan Teori Atom Dalton? |
| |
|
Which of the following is not a statement of Dalton's Atomic Theory? |
| |
|
|
| |
|
| A. |
|
Zarah terkecil jirim ialah atom |
| |
|
The smallest particle of matter is an atom |
| |
|
|
| B. |
|
Atom terdiri daripada sub-atom seperti neutron, proton dan elektron |
| |
|
Atoms are made up of sub-atoms such as neutrons, protons and electrons |
| |
|
|
| C. |
|
Atom tidak boleh dimusnahkan ataupun dicipta |
| |
|
Atoms cannot be destroyed or created |
| |
|
|
| D. |
|
Atom bagi unsur yang sama adalah serupa |
| |
|
The atoms of the same element are similar |
|
|
| |
| 2. |
|
Rajah 1 menunjukkan susunan zarah dalam tiga keadaan jirim pada suhu bilik. |
| |
|
Diagram 1 shows the arrangement of particles in three states of matter at room temperature. |
| |
|
|
| |
|
|
| |
|
Rajah 1 |
| |
|
Diagram 1 |
| |
|
|
| |
|
Apakah bahan P, Q dan R pada suhu bilik? |
| |
|
What are subtances P, Q and R at room temperature? |
| |
|
|
| |
|
| |
P |
Q |
R |
| A. |
Air
Water |
Glukosa
Glucose |
Hidrogen
Hydrogen |
| B. |
Glukosa
Glucose |
Hidrogen
Hydrogen |
Air
Water |
| C. |
Hidrogen
Hydrogen |
Air
Water |
Glukosa
Glucose |
| D. |
Air
Water |
Hidrogen
Hydrogen |
Glukosa
Glucose |
|
|
| |
| 3. |
|
Pernyataan berikut adalah ciri-ciri istimewa suatu unsur peralihan. |
| |
|
The following statements are the special characteristics of a transitional element. |
| |
|
|
| |
|
- Membentuk sebatian berwarna
Forming coloured compounds
- Mempunyai lebih daripada satu nombor pengoksidaan dalam sebatiannya
Has more than one oxidation number in its compound
|
|
| |
|
|
| |
|
Antara unsur berikut, yang manakah mempunyai sifat seperti yang ditunjukkan di atas? |
| |
|
Which of the following elements has the properties as shown above? |
| |
|
|
| |
|
| A. |
|
Boron |
| |
|
Boron |
| |
|
|
| B. |
|
Sulfur |
| |
|
Sulphur |
| |
|
|
| C. |
|
Kromium |
| |
|
Chromium |
| |
|
|
| D. |
|
Kalium |
| |
|
Potassium |
|
|
|
|
|
|
| 4. |
|
Antara berikut, yang manakah betul mengenai susunan unsur dalam Jadual Berkala Unsur? |
| |
|
Which of the following is correct regarding the arrangement of elements in the Periodic Table of Elements? |
| |
|
|
| |
|
| I. |
|
Kala unsur ditunjukkan oleh bilangan petala yang berisi dengan elektron unsur tersebut |
| |
|
The period of the element is indicated by the number of shells filled with electrons of the element |
| |
|
|
| II. |
|
Unsur-unsur disusun mengikut urutan nombor proton yang meningkat secara mendatar |
| |
|
Elements are arranged according to the order of proton number which increases horizontally |
| |
|
|
| III. |
|
Untuk elektron valens di antara nombor 3 dan 8, kumpulan itu ditentukan dengan menambahkan 20 kepada bilangan elektron valens |
| |
|
For valence electron in between the number 3 and 8, the group is determined by adding 20 to the number of valence electrons |
| |
|
|
| IV. |
|
Untuk elektron valens di antara nombor 3 dan 8, kumpulan itu ditentukan dengan menambahkan 10 kepada bilangan elektron valens |
| |
|
For valence electron in between the number 3 and 8, the group is determined by adding 10 to the number of valence electrons |
| |
|
|
| A. |
|
I, II dan III |
| |
|
I, II and III |
| |
|
|
| B. |
|
II, III dan IV |
| |
|
II, III and IV |
| |
|
|
| C. |
|
I, II dan IV |
| |
|
I, II and IV |
| |
|
|
| D. |
|
I, III dan IV |
| |
|
I, III and IV |
|
|
| |
| 5. |
|
Antara berikut, yang manakah betul mengenai perubahan sifat unsur merentasi kala dalam Jadual Berkala Unsur? |
| |
|
Which of the following is the correct change of property of elements across the period in the Periodic Table of Elements? |
| |
|
|
| |
|
| A. |
|
Jejari atom meningkat |
| |
|
The atomic radius increases |
| |
|
|
| B. |
|
Jisim atom relatif berkurang |
| |
|
The relative atomic mass decreases |
| |
|
|
| C. |
|
Bilangan proton dalam setiap atom meningkat |
| |
|
The number of protons for each atom increases |
| |
|
|
| D. |
|
Bilangan elektron valens dalam setiap atom berkurang |
| |
|
The number of valence electrons in each atom decreases |
|
|
| |
| 6. |
|
Sebatian manakah yang terbentuk melalui pemindahan elektron? |
| |
|
Which compound is formed by transferring electrons? |
| |
|
|
| |
|
| A. |
|
Oksigen |
| |
|
Oxygen |
| |
|
|
| B. |
|
Karbon dioksida |
| |
|
Carbon dioxide |
| |
|
|
| C. |
|
Natrium klorida |
| |
|
Sodium chloride |
| |
|
|
| D. |
|
Hidrogen peroksida |
| |
|
Hydrogen peroxide |
|
|
|
|
|
|
| 7. |
|
Antara berikut, manakah unsur yang berada di dalam Kumpulan 18 Jadual Berkala Unsur? |
| |
|
Which of the following elements are in Group 18 in the Periodic Table of Elements? |
| |
|
|
| |
|
| A. |
|
Argon dan xenon |
| |
|
Argon and xenon |
| |
|
|
| B. |
|
Hidrogen dan oksigen |
| |
|
Hydrogen and oxygen |
| |
|
|
| C. |
|
Oksigen dan xenon |
| |
|
Oxygen and xenon |
| |
|
|
| D. |
|
Argon dan hidrogen |
| |
|
Argon and hydrogen |
|
|
| |
| 8. |
|
Elektrik adalah salah satu tenaga yang penting dalam kehidupan seharian. Industri yang menjana tenaga elektrik menggunakan sifat isotop bagi meningkatkan kecekapan penghasilannya. Apakah isotop yang digunakan itu? |
| |
|
Electric is one of the important energy that we use in our daily life. The industry of generating electricity makes use of isotope features to increase its efficiency. What is the isotope used for this industry? |
| |
|
|
| |
|
| A. |
|
Karbon-14 |
| |
|
Carbon-14 |
| |
|
|
| B. |
|
Uranium-238 |
| |
|
Uranium-238 |
| |
|
|
| C. |
|
Kobalt-60 |
| |
|
Cobalt-60 |
| |
|
|
| D. |
|
Uranium-235 |
| |
|
Uranium-235 |
|
| |
|
| 9. |
|
Rubidium terletak di bawah kalium dalam Jadual Berkala. Antara pernyataan berikut, yang manakah benar mengenai rubidium? |
| |
|
Rubidium is located below potassium in the Periodic Table. Which of the following statement is true about rubidium? |
| |
|
|
| |
|
| A. |
|
Rubidium kurang elektropositif daripada kalium |
| |
|
Rubidium is less electropositive than potassium |
| |
|
|
| B. |
|
Atom rubidium lebih kecil daripada atom kalium |
| |
|
Rubidium atom is smaller than potassium atom |
| |
|
|
| C. |
|
Apabila rubidium ditindak balas dengan air dan diuji dengan kertas litmus biru, kertas litmus itu bertukar menjadi merah |
| |
|
When rubidium is reacted with water and tested with blue litmus paper, the litmus paper turns red |
| |
|
|
| D. |
|
Rubidium lebih reaktif berbanding dengan litium |
| |
|
Rubidium is more reactive compared to lithium |
|
|
| |
| 10. |
|
Apabila merentasi Kala 3, sifat oksida berubah daripada |
| |
|
When going across Period 3, from left to right, the oxide properties changes from |
| |
|
|
| |
|
| A. |
|
asid, kepada amfoterik dan kemudian bes |
| |
|
acidic, to amphoteric and then to basic |
| |
|
|
| B. |
|
bes, kepada amfoterik dan kemudian asid |
| |
|
basic, to amphoteric and then to acidic |
| |
|
|
| C. |
|
amfoterik, kepada bes dan kemudian asid |
| |
|
amphoteric, to basic and then to acidic |
| |
|
|
| D. |
|
bes, kepada asid dan kemudian amfoterik |
| |
|
basic, to acidic and then to amphoteric |
|
|
|
|
|
|
| 11. |
|
Antara berikut, yang manakah sifat magnesium klorida? |
| |
|
Which of the following are the properties of magnesium chloride? |
| |
|
|
| |
|
| I. |
|
Mudah meruap |
| |
|
Volatile |
| |
|
|
| II. |
|
Larut dalam pelarut organik |
| |
|
Soluble in an organic solvent |
| |
|
|
| III. |
|
Takat lebur yang tinggi |
| |
|
High melting point |
| |
|
|
| IV. |
|
Mengalirkan elektrik dalam keadaan lebur |
| |
|
Conducts electricity in the molten state |
| |
|
|
| A. |
|
I dan II |
| |
|
I and II |
| |
|
|
| B. |
|
III dan IV |
| |
|
III and IV |
| |
|
|
| C. |
|
I dan III |
| |
|
I and III |
| |
|
|
| D. |
|
II dan IV |
| |
|
II and IV |
|
|
| |
| 12. |
|
Manakah antara pemerhatian berikut membuktikan benzena adalah satu sebatian kovalen? |
| |
|
Which of the following observations proves that benzene is a covalent compound? |
| |
|
|
| |
|
| I. |
|
Ia mempunyai takat lebur yang rendah |
| |
|
It has a low melting point |
| |
|
|
| II. |
|
Ia tidak mengkonduksi elektrik dalam keadaan cecair |
| |
|
It does not conduct electricity in the liquid state |
| |
|
|
| III. |
|
Ia boleh larut dalam pelarut organik tetapi tidak dalam air |
| |
|
It is soluble in organic solvents but not in water |
| |
|
|
| IV. |
|
la adalah satu pelarut bagi natrium klorida |
| |
|
It is a solvent for sodium chloride |
| |
|
|
| A. |
|
I, II dan III |
| |
|
I, II and III |
| |
|
|
| B. |
|
I, II dan IV |
| |
|
I, II and IV |
| |
|
|
| C. |
|
II, III dan IV |
| |
|
II, III and IV |
| |
|
|
| D. |
|
I, III dan IV |
| |
|
I, III and IV |
|
|
|
|
|
|
| 13. |
|
Antara berikut, yang manakah benar mengenai hidrogen klorida yang dilarutkan dalam air? |
| |
|
Which of the following is true about hydrogen chloride that is dissolved in water? |
| |
|
|
| |
|
| I. |
|
Ion hidroksida terbentuk dalam larutan |
| |
|
Hydroxide ion is formed in the solution |
| |
|
|
| II. |
|
Ion hidrogen terbentuk dalam larutan |
| |
|
Hydroxonium ion is formed in the solution |
| |
|
|
| III. |
|
Larutan ini menyebabkan kertas litmus biru berubah menjadi merah |
| |
|
The solution shows turns blue litmus paper to red |
| |
|
|
| IV. |
|
Larutan ini menyebabkan kertas litmus merah berubah menjadi biru |
| |
|
The solution shows turns red litmus paper to blue |
| |
|
|
| A. |
|
II dan IV |
| |
|
I and IV |
| |
|
|
| B. |
|
II dan III |
| |
|
II and III |
| |
|
|
| C. |
|
I dan III |
| |
|
I and III |
| |
|
|
| D. |
|
I dan IV |
| |
|
I and IV |
|
|
| |
| 14. |
|
Garam berikut yang manakah larut dalam air? |
| |
|
Which of the following salt is soluble in water? |
| |
|
|
| |
|
| A. |
|
Kalsium karbonat |
| |
|
Calcium carbonate |
| |
|
|
| B. |
|
Natrium nitrat |
| |
|
Sodium nitrate |
| |
|
|
| C. |
|
Barium sulfat |
| |
|
Barium sulphate |
| |
|
|
| D. |
|
Argentum klorida |
| |
|
Silver chloride |
|
|
| |
| 15. |
|
Antara berikut, pasangan yang manakah tidak benar? |
| |
|
Which of the following pair is not correct? |
| |
|
|
| |
|
| |
Tindak balas
Reaction |
Kelajuan
Speed |
| A. |
Pengaratan
Rusting |
Perlahan
Slow |
| B. |
Hakisan pada batu
Erosion of rock |
Perlahan
Slow |
| C. |
Pembakaran bahan api
Combustion of fuel |
Cepat
Fast |
| D. |
Peneutralan asid dan alkali
Neutralisation of acid and alkali |
Perlahan
Slow |
|
|
|
|
|
|
| 16. |
|
Apakah maksud kadar tindak balas seketika? |
| |
|
What is the meaning of instantaneous rate of reaction? |
| |
|
|
| |
|
| A. |
|
Kepantasan tindak balas berlaku dalam satu jangka masa |
| |
|
The speed of reaction progress over an interval of time |
| |
|
|
| B. |
|
Kadar tindak balas yang berlaku pada mana-mana masa |
| |
|
Rate of reaction that happens at any given time |
| |
|
|
| C. |
|
Kadar tindak balas yang berlaku hanya seketika |
| |
|
Rate of reaction that happens in an instant |
| |
|
|
| D. |
|
Kadar tindak balas yang sangat tinggi |
| |
|
Rate of reaction is very high |
| |
|
|
|
|
| 17. |
|
Manakah antara berikut adalah benar mengenai kegunaan neoprena (polikloroprena)? |
| |
|
Which of the following is true about the uses of neoprene (polychloroprene)? |
| |
|
|
| |
|
| A. |
|
Implan perubatan |
| |
|
Medical implants |
| |
|
|
| B. |
|
Alatan memasak |
| |
|
Cooking utensils |
| |
|
|
| C. |
|
Komponen automotif |
| |
|
Automotive components |
| |
|
|
| D. |
|
Tali sawat |
| |
|
Conveyor belts |
|
|
|
| 18. |
|
Rajah 2 menunjukkan sel elektrolitik. |
| |
|
Diagram 2 shows an electrolytic cell. |
| |
|
|
| |
|
|
| |
|
Rajah 2 |
| |
|
Diagram 2 |
| |
|
|
| |
|
Antara berikut, yang manakah Bahan X yang sesuai supaya mentol menyala? |
| |
|
Which of the following is the suitable Material X so that the light bulb lights up? |
| |
|
|
| |
|
| A. |
|
Naftalena pepejal |
| |
|
Solid naphthalene |
| |
|
|
| B. |
|
Magnesium oksida pepejal |
| |
|
Solid magnesium oxide |
| |
|
|
| C. |
|
Magnesium oksida leburan |
| |
|
Molten magnesium oxide |
| |
|
|
| D. |
|
Sulfur leburan |
| |
|
Molten sulphur |
|
|
|
|
|
|
| 19. |
|
Rajah 3 menunjukkan susunan elektron dalam sebatian TZ. |
| |
|
Diagram 3 shows the electron arrangement for compound TZ. |
| |
|
|
| |
|
|
| |
|
Rajah 3 |
| |
|
Diagram 3 |
| |
|
|
| |
|
Unsur manakah yang diwakili oleh T and Z? |
| |
|
Which elements are represented by T and Z? |
| |
|
|
| |
|
| |
T |
Z |
| A. |
Natrium
Sodium |
Oksigen
Oxygen |
| B. |
Magnesium
Magnesium |
Oksigen
Oxygen |
| C. |
Natrium
Sodium |
Florin
Fluorine |
| D. |
Magnesium
Magnesium |
Florin
Fluorine |
|
|
| |
| 20. |
|
Antara berikut, yang manakah berlaku dalam tindak balas penurunan? |
| |
|
Which of the following occurs in reduction reaction? |
| |
|
|
| |
|
| A. |
|
Kehilangan oksigen |
| |
|
Loss of oxygen |
| |
|
|
| B. |
|
Kehilangan hidrogen |
| |
|
Loss of hydrogen |
| |
|
|
| C. |
|
Kehilangan elektron |
| |
|
Loss of electrons |
| |
|
|
| D. |
|
Penambahan nombor pengoksidaan |
| |
|
Increase in oxidation number |
|
|
| |
| 21. |
|
Apakah maksud siri homolog? |
| |
|
What is the meaning of the homologous series? |
| |
|
|
| |
|
| A. |
|
Sekumpulan sebatian dengan sifat kimia yang serupa |
| |
|
Compounds grouped together for their similar chemical properties |
| |
|
|
| B. |
|
Fenomena suatu sebatian yang mempunyai formula molekul sama tetapi dua atau lebih formula struktur yang berbeza |
| |
|
A phenomenon where a compound has the same molecular formula but with two or more different structural formulae |
| |
|
|
| C. |
|
Sumber tenaga selain bahan api fosil yang tidak dapat diperbaharui |
| |
|
To alternative energy sources other than non-renewable fossil fuels |
| |
|
|
| D. |
|
Sebatian yang mengandungi karbon sebagai unsur juzuknya |
| |
|
Compounds that contain carbon as their constituent element |
|
|
|
|
|
|
| 22. |
|
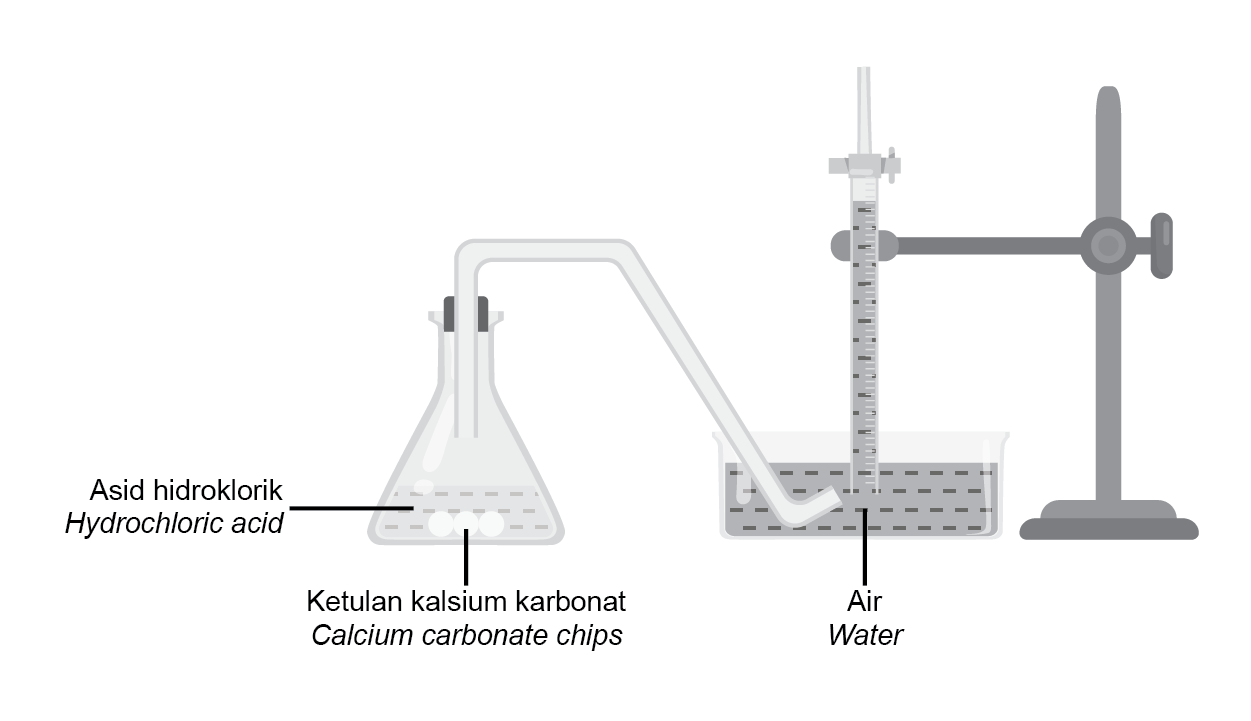
Rajah 4 menunjukkan susunan radas bagi mengkaji kadar tindak balas antara kalsium karbonat dan asid hidroklorik. |
| |
|
Diagram 4 shows the apparatus set-up used to study the rate of reaction of calcium carbonate and hydrochloric acid. |
| |
|
|
| |
|
 |
| |
|
|
| |
|
Rajah 4 |
| |
|
Diagram 4 |
| |
|
|
| |
|
Kadar tindak balas boleh ditingkatkan dengan |
| |
|
The rate of reaction can be increased by |
| |
|
|
| |
|
| A. |
|
mengisar kalsium karbonat |
| |
|
grinding the calcium carbonate |
| |
|
|
| B. |
|
merendahkan suhu asid hidroklorik |
| |
|
lowering the temperature of hydrochloric acid |
| |
|
|
| C. |
|
menggunakan kelalang kon yang lebih besar |
| |
|
using a larger conical flask |
| |
|
|
| D. |
|
menambahkan air kepada asid hidroklorik |
| |
|
adding water to hydrochloric acid |
|
|
| |
| 23. |
|
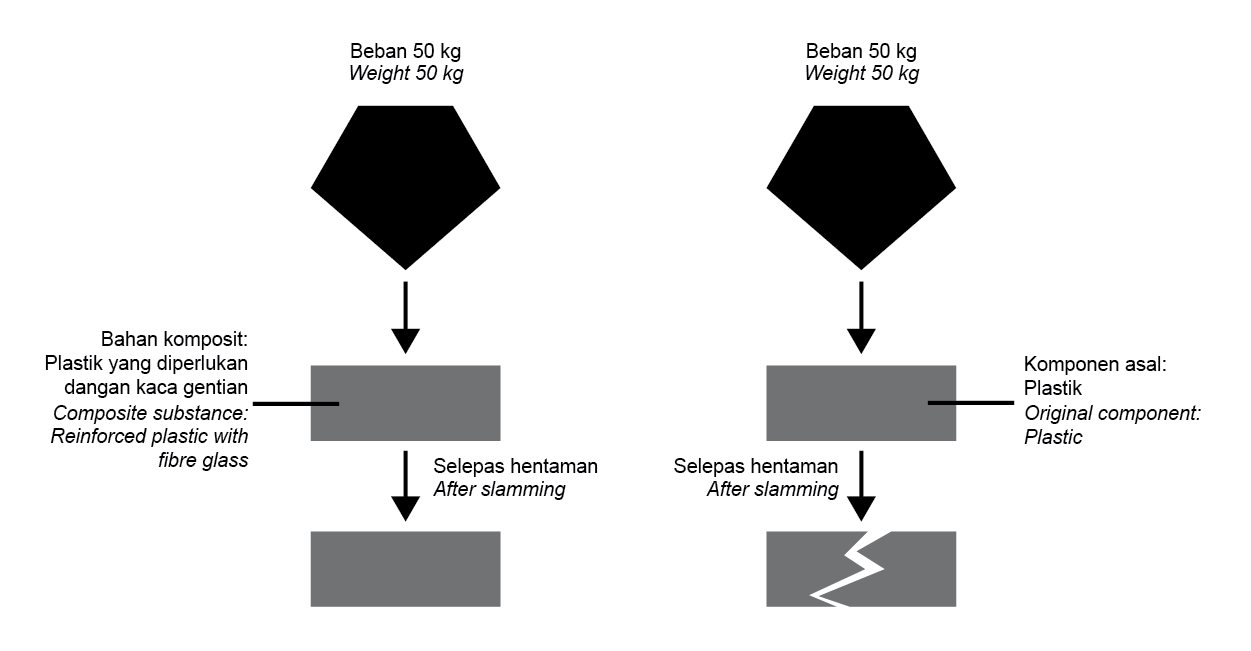
Rajah 5 menunjukkan kesan pemberat yang dijatuhkan ke atas bahan komposit dan komponen asalnya. |
| |
|
Diagram 5 shows the effect of a weight that is dropped onto a composite substance and its original components. |
| |
|
|
| |
|
 |
| |
|
|
| |
|
Rajah 5 |
| |
|
Diagram 5 |
| |
|
|
| |
|
Apakah ciri bahan komposit tersebut? |
| |
|
What is the characteristic of the composite substance? |
| |
|
|
| |
|
| A. |
|
Kuat dan keras |
| |
|
Strong and hard |
| |
|
|
| B. |
|
Keras dan mulur |
| |
|
Hard and ductile |
| |
|
|
| C. |
|
Kuat dan kenyal |
| |
|
Strong and elastic |
| |
|
|
| D. |
|
Kenyal dan mulur |
| |
|
Elastic and ductile |
|
|
|
|
|
|
| 24. |
|
|
Sebatian X merupakan suatu hidrokarbon tak tepu manakala sebatian Y merupakan satu hidrokarbon tepu.
Compound X is an unsaturated hydrocarbon while compound Y is a saturated hydrocarbon.
|
|
| |
|
|
| |
|
Antara perbandingan berikut, yang manakah benar tentang sebatian X dan Y? |
| |
|
Which of the following comparison is true of compound X and Y? |
| |
|
|
| |
|
| A. |
|
Sebatian Y hanya mempunyai ikatan kovalen tunggal |
| |
|
Compound Y only has single covalent bonds |
| |
|
|
| B. |
|
Peratusan karbon menurut jisim dalam Y adalah lebih tinggi |
| |
|
The percentage of carbon by mass in Y is higher |
| |
|
|
| C. |
|
Molekul X mempunyai bilangan atom hidrogen yang lebih banyak |
| |
|
Molecule X has a greater number of hydrogen atoms |
| |
|
|
| D. |
|
Sebatian Y mempunyai ikatan ganda dua manakala sebatian X tidak mempunyai ikatan ganda dua |
| |
|
Compound Y has a double bond while compound X does not have a double bond |
|
|
| |
| 25. |
|
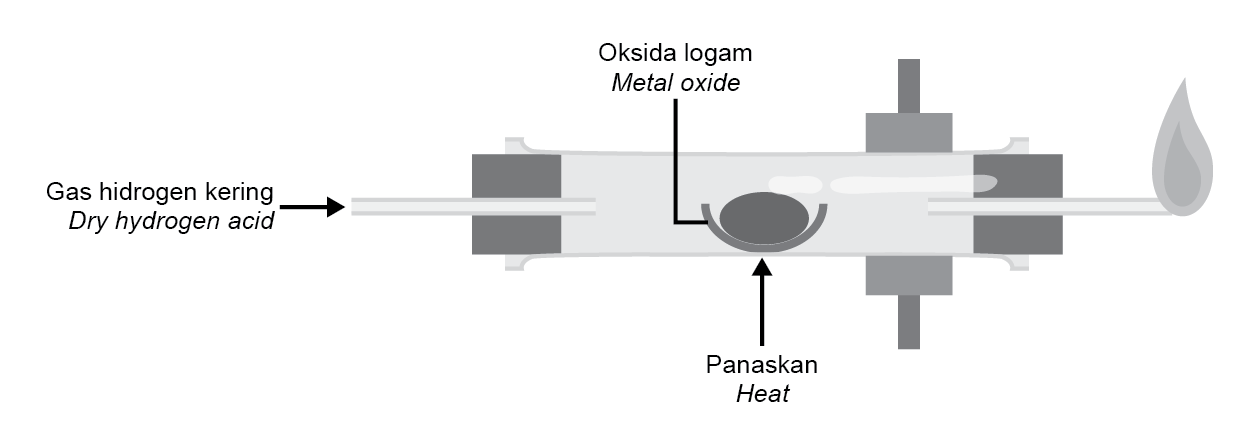
Rajah 6 menunjukkan susunan radas untuk menentukan formula empirik suatu logam oksida. |
| |
|
Diagram 6 shows the set-up of the apparatus to determine the empirical formula of a metal oxide. |
| |
|
|
| |
|
 |
| |
|
|
| |
|
Rajah 6 |
| |
|
Diagram 6 |
| |
|
|
| |
|
Antara logam oksida berikut, yang manakah sesuai digunakan dalam rajah? |
| |
|
Which of the following metal oxides is suitable to be used in the diagram? |
| |
|
|
| |
|
| A. |
|
Zink oksida |
| |
|
Zinc oxide |
| |
|
|
| B. |
|
Ferum oksida |
| |
|
Iron oxide |
| |
|
|
| C. |
|
Aluminium oksida |
| |
|
Aluminium oxide |
| |
|
|
| D. |
|
Magnesium oksida |
| |
|
Magnesium oxide |
|
|
| |
| 26. |
|
Pilihan yang manakah menunjukkan bahan matriks dan bahan pengukuhan yang betul untuk bahan komposit? |
| |
|
Which option shows the correct matrix substance and strengthening substance for the respective composite material? |
| |
|
|
| |
|
| |
Bahan komposit
Composite material |
Bahan matriks
Matrix substance |
Bahan pengukuhan
Strengthening substance |
| A. |
Konkrit diperkukuhkan
Reinforced concrete |
Jejaring dawai
Wire mesh |
Konkrit
Concrete |
| B. |
Gentian optikal
Optical fibre |
Gentian kaca silika
Silica glass fibres |
Kaca atau plastik
Glass or plastic |
| C. |
Kaca gentian
Fibre glass |
Plastik
Plastic |
Gentian kaca
Glass fibres |
| D. |
Kaca fotokromik
Photochromic glass |
AgCl dan CuCl
AgCl dan CuCl |
Kaca
Glass |
|
|
|
|
|
|
| 27. |
|
Jadual 1 menunjukkan susunan elektron unsur X dan Y. |
| |
|
Table 1 shows the electron arrangement of elements X and Y. |
| |
|
|
| |
|
Unsur X
Element X |
Unsur Y
Element Y |
| 2.8.3 |
2.6 |
|
| |
|
Jadual 1 |
| |
|
Table 1 |
| |
|
|
| |
|
Penyataan manakah betul tentang sebatian yang terbentuk daripada tindak balas antara X dan Y? |
| |
|
Which statement is correct about the compound formed from the reaction between X and Y? |
| |
|
|
| |
|
| A. |
|
Tidak larut dalam air |
| |
|
Insoluble in water |
| |
|
|
| B. |
|
Boleh mengalirkan arus elektrik |
| |
|
Can conduct electricity |
| |
|
|
| C. |
|
Larut dalam pelarut organik |
| |
|
Soluble in an organic solvent |
| |
|
|
| D. |
|
Takat lebur dan takat didih yang tinggi |
| |
|
High melting and boiling point |
|
|
| |
| 28. |
|
Persamaan berikut mewakili tindak balas antara magnesium oksida dengan asid nitrik. |
| |
|
The following equation represents the reaction between magnesium oxide and nitric acid. |
| |
|
|
| |
|
\(MgO + 2HNO_3 \rightarrow Mg(NO_3)_2 + H_2O\) |
| |
|
|
| |
|
Magnesium oksida yang berlebihan bertindak balas dengan 50 cm3 asid nitrik 2.0 mol dm–3. Apakah jisim maksimum garam magnesium nitrat yang terbentuk? |
| |
|
Excess magnesium oxide is reacted with 50 cm3 of 2.0 mol dm–3 nitric acid . What is the maximum mass of magnesium nitrate salt formed? |
| |
|
|
| |
|
[Jisim atom relatif: N = 14, O= 16, Mg= 24] |
| |
|
[Relative atomic mass: N = 14, O= 16, Mg= 24] |
| |
|
|
| |
|
| A. |
|
7.40 g |
| |
|
|
| B. |
|
6.30g |
| |
|
|
| C. |
|
4.30 g |
| |
|
|
| D. |
|
14.80 g |
|
|
| |
| 29. |
|
Rajah 7 menunjukkan sejenis unsur. |
| |
|
Diagram 7 shows a kind of element. |
| |
|
|
| |
|
| |
\(\begin{array}{|l|} \hline 31\\ \hspace{3mm}\Huge{Y}\\ 15\\ \hline \end{array}\) |
|
|
| |
|
Rajah 7 |
| |
|
Diagram 7 |
| |
|
|
| |
|
Berdasarkan rajah di atas, yang manakah menunjukkan kedudukan unsur Y yang betul dalam Jadual Berkala Unsur? |
| |
|
Based on the diagram above, which one shows the correct position of element Y in the Periodic Table of Elements? |
| |
|
|
| |
|
| |
Kala
Period |
Kumpulan
Group |
| A. |
5 |
3 |
| B. |
5 |
15 |
| C. |
3 |
15 |
| D. |
3 |
16 |
|
|
|
|
|
|
| 30. |
|
Rajah 8 menunjukkan susunan elektron atom unsur X, Y dan Z. |
| |
|
Diagram 8 shows the electron arrangement of the atom of elements X, Y and Z. |
| |
|
|
| |
|
|
| |
|
Rajah 8 |
| |
|
Diagram 8 |
| |
|
|
| |
|
Antara berikut, yang manakah tertib menurun yang betul bagi saiz atom unsur-unsur ini? |
| |
|
Which of the following is the correct descending order of the atomic size of these elements? |
| |
|
|
| |
|
| A. |
|
Z, X, Y |
| |
|
|
| B. |
|
X, Y, Z |
| |
|
|
| C. |
|
Y, X, Z |
| |
|
|
| D. |
|
X, Z, Y |
|
|
| |
| 31. |
|
Jadual 2 menunjukan kepekatan dan isi padu bagi dua jenis asid kuat yang berlainan, X dan Y, yang digunakan untuk meneutralkan 20.0 cm3 larutan natrium hidroksida 0.5 mol dm–3. |
| |
|
Table 2 shows the concentration and volume of two different types of strong acids, X and Y, which are used to neutralise 20.0 cm3 of 0.5 mol dm–3 sodium hydroxide solution. |
| |
|
|
| |
|
Asid
Acid |
X |
Y |
Kepekatan
Concentration |
0.5 mol dm–3 |
0.5 mol dm–3 |
Isi padu
Volume |
V cm3 |
2V cm3 |
|
| |
|
Jadual 2 |
| |
|
Table 2 |
| |
|
|
| |
|
Berdasarkan Jadual 2, apakah asid X dan asid Y? |
| |
|
Based on the Table 2, what are acid X and acid Y? |
| |
|
|
| |
|
| |
Asid X
Acid X |
Asid Y
Acid Y |
| A. |
Asid hidroklorik
Hydrochloric acid |
Asid sulfurik
Sulphuric acid |
| B. |
Asid sulfurik
Sulphuric acid |
Asid hidroklorik
Hydrochloric acid |
| C. |
Asid hidroklorik
Hydrochloric acid |
Asid nitrik
Nitric acid |
| D. |
Asid nitrik
Nitric acid |
Asid etanoik
Ethanoic acid |
|
|
|
|
|
|
| 32. |
|
Apakah perubahan yang boleh diperhatikan berdasarkan persamaan berikut? |
| |
|
What are the observable changes based on the following equation? |
| |
|
|
| |
|
\(CaCO_3(p)+2HCl(ak)\rightarrow CaCl_2(ak) + H_2O(ce)+CO_2(g)\) |
| |
|
|
| |
|
| I. |
|
Pertambahan jisim kalsium karbonat |
| |
|
Increase in mass of calcium carbonate |
| |
|
|
| II. |
|
Pengurangan jisim kalsium karbonat |
| |
|
Decrease in the mass of calcium carbonate |
| |
|
|
| III. |
|
Peningkatan isi padu gas karbon dioksida |
| |
|
Increase in the volume of carbon dioxide gas |
| |
|
|
| IV. |
|
Pengurangan isi padu kalsium klorida |
| |
|
Decrease in volume of calcium chloride |
| |
|
|
| A. |
|
I dan II |
| |
|
I and II |
| |
|
|
| B. |
|
II dan IV |
| |
|
II and IV |
| |
|
|
| C. |
|
I dan III |
| |
|
I and III |
| |
|
|
| D. |
|
II dan III |
| |
|
II and III |
|
|
| |
| 33. |
|
Pak Ali ialah seorang penoreh getah. Setelah beberapa jam menoreh pokok getahnya, Pak Ali mengumpulkan lateks itu dan membawanya ke kilang. Dia mendapati bahawa lateks telah menggumpal sebelum dia sampai ke kilang. Apa yang perlu dilakukan oleh Pak Ali untuk mengelakkan lateks itu daripda menggumpal? |
| |
|
Pak Ali is a rubber tapper. After a few hours tapping his rubber tree, Pak Ali collected the latex and brought it to the factory. He found that the latex coagulated before he reaches the factory. What should Pak Ali do to prevent the latex from coagulating? |
| |
|
|
| |
|
| A. |
|
Tambah asid formik ke dalam lateks |
| |
|
Add formic acid into the latex |
| |
|
|
| B. |
|
Tambah larutan natrium klorida ke dalam lateks |
| |
|
Add sodium chloride solution into the latex |
| |
|
|
| C. |
|
Tambah larutan ammonia ke dalam lateks |
| |
|
Add ammonia solution into the latex |
| |
|
|
| D. |
|
Masukkan air ke dalam lateks |
| |
|
Add water into the latex |
|
|
|
|
|
|
| 34. |
|
Rajah 9 menunjukkan graf kepekatan terhadap masa. |
| |
|
Diagram 9 shows the graph of concentration against time. |
| |
|
|
| |
|
|
| |
|
Rajah 9 |
| |
|
Diagram 9 |
| |
|
|
| |
|
Antara berikut, yang manakah betul mengenai graf ini? |
| |
|
Which of the following are true regarding the graph? |
| |
|
|
| |
|
| I. |
|
Kepekatan bahan tindak balas menurun dan kepekatan hasil meningkat apabila masa bertambah |
| |
|
The concentration of reactant decreases and the concentration of product increases as the time increases |
| |
|
|
| II. |
|
Kepekatan bahan tindak balas meningkat dan kepekatan hasil meningkat apabila masa bertambah |
| |
|
The concentration of reactant increases and the concentration of product increases as the time increases |
| |
|
|
| III. |
|
Kepekatan bahan tindak balas berkadar songsang terhadap masa |
| |
|
The concentration of reactant is inversely proportional to the time |
| |
|
|
| IV. |
|
Kepekatan hasil tindak balas berkadar songsang terhadap masa |
| |
|
The concentration of the product is inversely proportional to the time |
| |
|
|
| A. |
|
I dan III |
| |
|
I and III |
| |
|
|
| B. |
|
II dan III |
| |
|
II and III |
| |
|
|
| C. |
|
II dan IV |
| |
|
II and IV |
| |
|
|
| D. |
|
I dan IV |
| |
|
I and IV |
|
|
|
|
|
|
| 35. |
|
Rajah 10 menunjukkan penguraian pepejal X apabila dipanaskan. |
| |
|
Diagram 10 shows the decomposition of solid X when heated. |
| |
|
|
| |
|
|
| |
|
Rajah 10 |
| |
|
Diagram 10 |
| |
|
|
| |
|
Baki Y berwarna perang ketika panas dan kuning ketika sejuk. Gas Z menukar air kapur menjadi keruh. Antara berikut, yang manakah pepejal X, baki Y dan gas Z? |
| |
|
The colour of residue Y is brown when hot and yellow when cold. Gas Z turns limewater cloudy. Which of the following is solid X, residue Y and gas Z? |
| |
|
|
| |
|
| |
Pepejal X
Solid X |
Baki Y
Residue Y |
Gas Z
Gas Z |
| A. |
Plumbum(II) oksida
Lead(II) oxide |
Plumbum(II) karbonat
Lead(II) carbonate |
Karbon dioksida
Carbon dioxide |
| B. |
Plumbum(II) karbonat
Lead(II) carbonate |
Plumbum(II) oksida
Lead(II) oxide |
Karbon dioksida
Carbon dioxide |
| C. |
Plumbum(II) karbonat
Lead(II) carbonate |
Plumbum(II) oksida
Lead(II) oxide |
Oksigen
Oxygen |
| D. |
Zink(II) karbonat
Zinc(II) carbonate |
Zink(II) oksida
Zinc(II) oxide |
Karbon dioksida
Carbon dioxide |
|
|
| |
| 36. |
|
Rajah 11 menunjukkan gambar rajah aras tenaga bagi suatu tindak balas. |
| |
|
Diagram 11 shows the energy level diagram of a reaction. |
| |
|
|
| |
|
|
| |
|
Rajah 11 |
| |
|
Diagram 11 |
| |
|
|
| |
|
Antara A, B, C dan D, yang manakah mewakili tenaga pengaktifan bagi tindak balas itu? |
| |
|
Which of the following A, B, C and D represents the activation energy of the reaction? |
|
|
|
|
|
| 37. |
|
Rajah 12 menunjukkan susunan radas bagi satu eksperimen untuk menentukan kadar tindak balas dengan mencatatkan masa markah “X' menghilang. |
| |
|
Figure 12 shows the apparatus set-up for an experiment to determine the rate of reaction by recording the time as soon as the ‘X’ mark disappears. |
| |
|
|
| |
|
|
| |
|
Rajah 12 |
| |
|
Diagram 12 |
| |
|
|
| |
|
Apakah bahan X dan Y supaya kadar tindak balas boleh ditentukan? |
| |
|
What are substances X and Y in order that the rate of reaction can be determined? |
| |
|
|
| |
|
| |
X |
Y |
| A. |
Asid hidroklorik
Hydrochloric acid |
Magnesium
Magnesium |
| B. |
Asid sulfurik
Sulphuric acid |
Sulphuric acid
Calcium carbonate |
| C. |
Asid hidroklorik
Hydrochloric acid |
Sodium thiosulphate
Sodium thiosulphate |
| D. |
Hidrogen peroksida
Hydrogen peroxide |
Hydrogen peroxide
Manganese(IV) oxide |
|
|
|
|
|
|
| 38. |
|
Rajah 13 menunjukkan satu sel kimia. |
| |
|
Diagram 13 shows a chemical cell. |
| |
|
|
| |
|
|
| |
|
Rajah 13 |
| |
|
Diagram 13 |
| |
|
|
| |
|
Apakah pemerhatian yang dicatatkan di terminal positif? |
| |
|
What is the observation recorded at the positive terminal? |
| |
|
|
| |
|
| A. |
|
Elektrod magnesium terhakis |
| |
|
The magnesium electrode eroded |
| |
|
|
| B. |
|
Magnesium termendap pada elektrod kuprum |
| |
|
Magnesium deposited at copper electrode |
| |
|
|
| C. |
|
Gelembung gas terhasil |
| |
|
Gas bubble is produced |
| |
|
|
| D. |
|
Elektrod kuprum terhakis |
| |
|
The copper electrode eroded |
|
|
| |
| |
| 39. |
|
Antara ubat berikut, yang manakah tidak sesuai dengan fungsinya? |
| |
|
Which of the following medicine does not match its function? |
| |
|
|
| |
|
| |
Ubat
Medicine |
Fungsi
Function |
| A. |
Amfetamin
Amphetamine |
Untuk merangsang aktiviti otak dan badan
To stimulate the activity of the brain and body |
| B. |
Kodeina
Codeine |
Melegakan kesakitan
To relieve pain |
| C. |
Aspirin
Aspirin |
Membunuh bakteria
To kill the bacteria |
| D. |
Barbiturat
Barbiturate |
Menenangkan minda penggunanya
To calm the mind of user |
|
|
|
| 40. |
|
Rajah 14 menunjukkan sel elektrolitik. |
| |
|
Diagram 14 shows an electrolytic cell. |
| |
|
|
| |
|
|
| |
|
Rajah 14 |
| |
|
Diagram 14 |
| |
|
|
| |
|
Berdasarkan rajah di atas, pernyataan yang manakah adalah benar? |
| |
|
Based on the diagram above, which of the following statements are true? |
| |
|
|
| |
|
| A. |
|
Logam zink termendap pada anod |
| |
|
Zinc metal is deposited at the anode |
| |
|
|
| B. |
|
Logam zink termendap pada katod |
| |
|
Zinc metal is deposited at the cathode |
| |
|
|
| C. |
|
Ion klorida diturunkan pada anod |
| |
|
Chloride ion is reduced at the anode |
| |
|
|
| D. |
|
Ion klorida diturunkan pada katod |
| |
|
Chloride ion is reduced at the cathode |
|
| |
KERTAS PEPERIKSAAN TAMAT
|
|
|
|