| |
|
|
| |
| Definition of Soap |
| Sodium or potassium fatty acid salts. |
|
| |
| Introduction to Soap |
- Soaps are sodium or potassium fatty acid salts.
- Soaps are produced from the neutralisation reaction between fatty acids and alkalis.
- Fatty acids are long-chain carboxylic acids.
- Sources of fatty acids can be obtained from natural esters in animal fats or vegetable oils.
- The general formula for soap is \(RCOO−Na^+\) or \(RCOO−K^+\).
- R is an alkyl group containing at least 8 carbon atoms.
- However, this alkyl group usually contains 12 to 20 carbon atoms.
- R consists of saturated or unsaturated hydrocarbons.
|
|
| |
| Examples of Soap |
| Soap |
Chemical Formula |
Fatty Acid |
Source |
| Sodium laurate |
CH3(CH2)10COONa |
CH3(CH2)10COOH Asid laurik
|
Coconut oil |
| Sodium palmitate |
CH3(CH2)14COONa |
CH3(CH2)14COONa Asid palmitik |
Palm oil |
|
| |
| Detergent |
- Detergents are sodium salts of sulphonic acids.
|
|
|
- The production of detergents began during the second world war owing to the lack of animal fats and vegetable oils.
- Detergents are non-soap cleaning agents.
- Detergents are sodium salts of sulphonic acids.
- Two types of sulphonic acids used to make detergents are alkyl sulphonic acid and alkylbenzene sulphonic acid.
- Detergents are usually made from synthetic sources, such as petroleum fractions.
|
|
| |
| Example of Detergents |
| Alkyl Sulphonic Acid |
Alkylbenzene Sulphonic Acid |
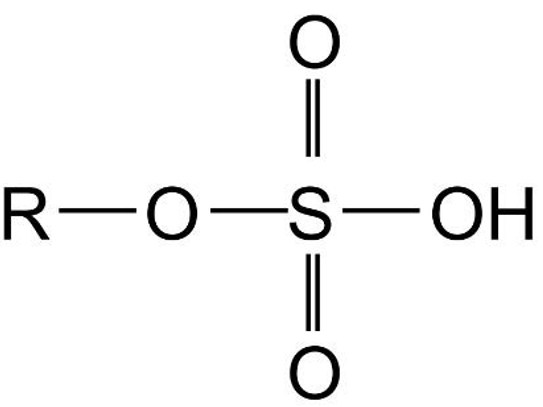 |
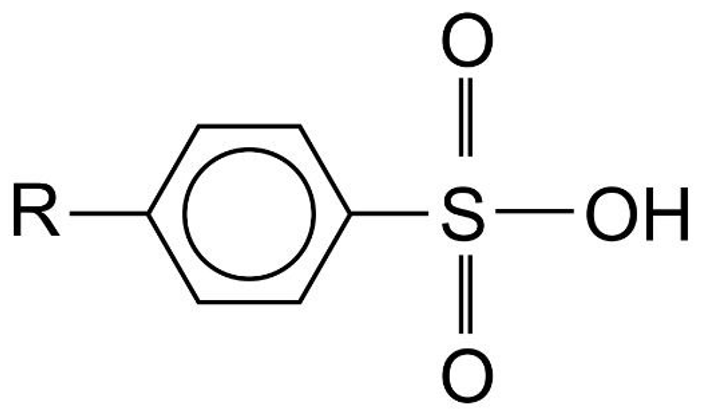 |
|
| |
| Prepartion of Soap |
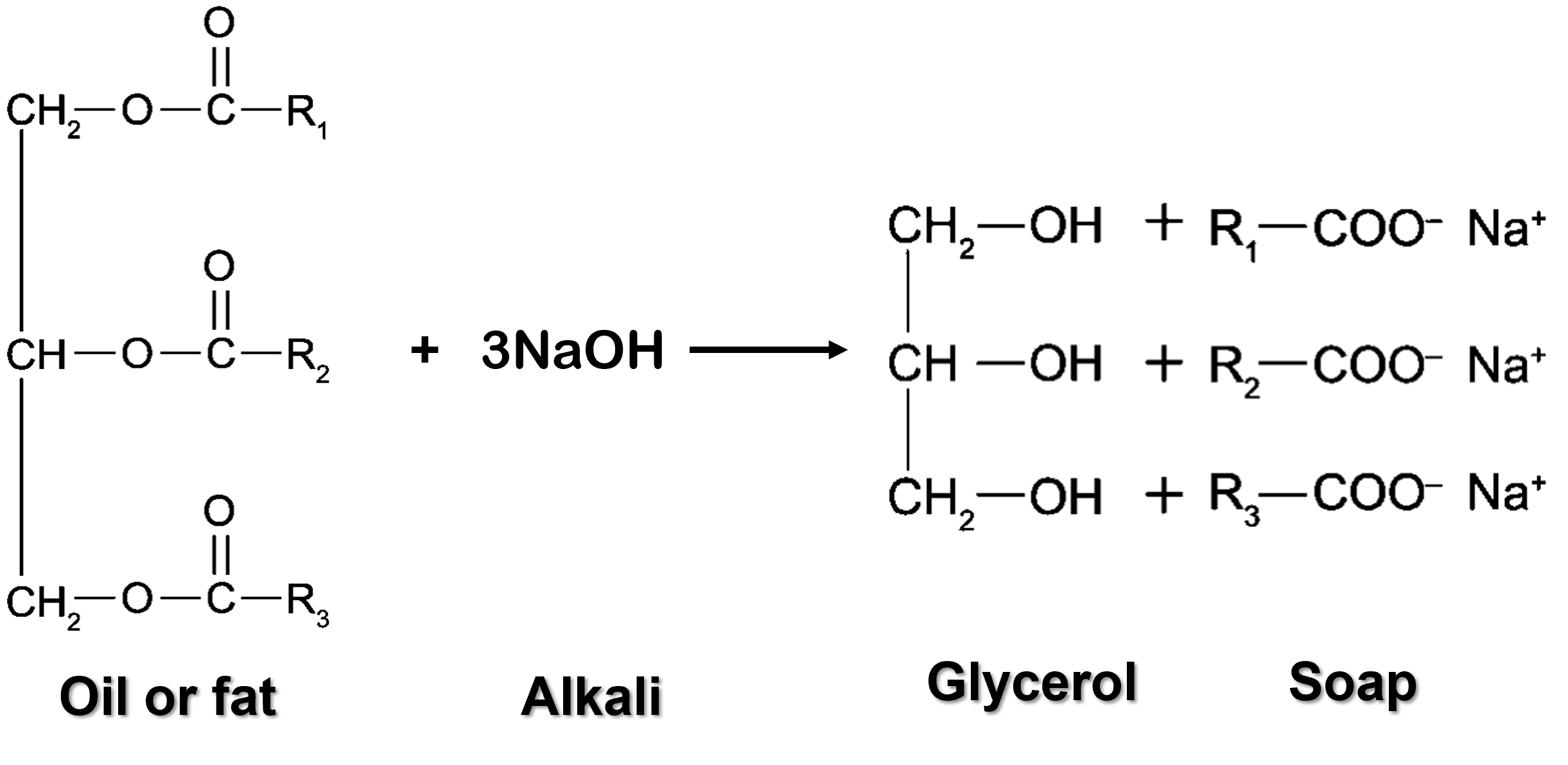
- Soaps can be prepared from natural sources through hydrolysis of oils or fats in sodium hydroxide, \(NaOH\) or potassium hydroxide, \(KOH\) solutions.
- This reaction is called saponification, which is the process of hydrolysis of oils or fats by alkalis.
- Oils or fats react with concentrated alkalis to produce glycerol and fatty acid salts, which is soap.
- Oils and fats are natural esters known as triglycerides.
|
|
| |
| General Equation of Saponification Reaction |
| Oil/Fat + Concentrated alkali → Soap + Glycerol |
 |
|
| |
| Prepartion of Detergents |
- Detergents are usually made from petroleum fractions and sulphuric acid, \(H_2SO_4\).
- They are produced through two processes which are:
- Sulphonation
- Neutralisation
|
|
| |
| Preparation of Sodium Alkylbenzene Sulphonate |
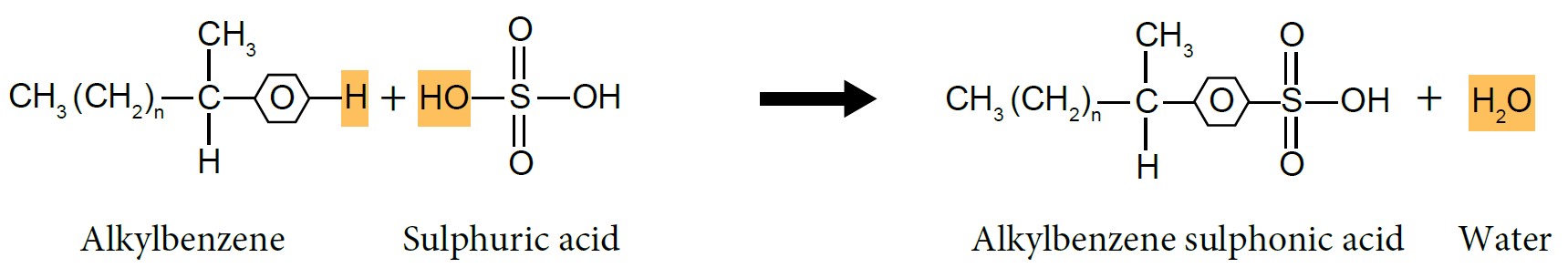
| (i) Sulphonation of Alkylbenzene |
- Alkylbenzene reacts with concentrated sulphuric acid, \(H_2SO_4\) to form alkylbenzene sulphonic acid.
|
- Alkylbenzene sulphonic acid will be neutralised by sodium hydroxide, NaOH solution to produce alkylbenzene sulphonate salt, which is detergent.
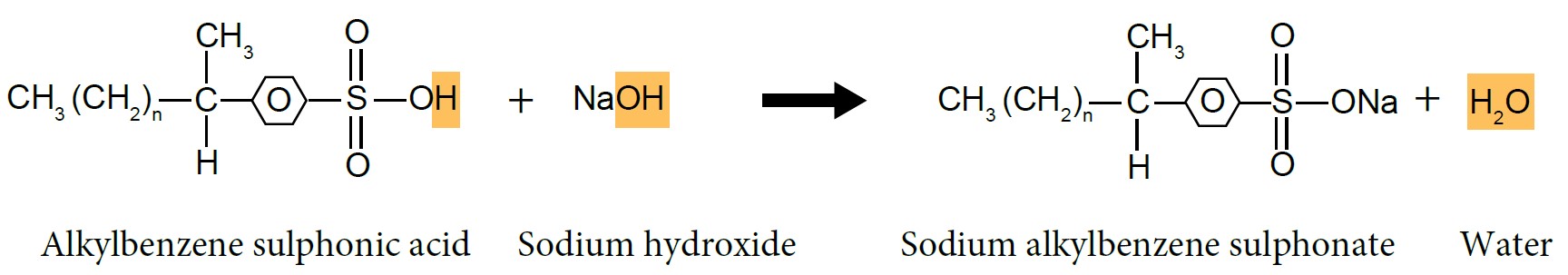
|
|
| |
| Preparation of Sodium Alkyl Sulphate |
| (i) Sulphonation of Alcohol |
- Long chain alcohol reacts with concentrated sulphuric acid, \(H_2SO_4\) to form alkyl sulphonic acid.
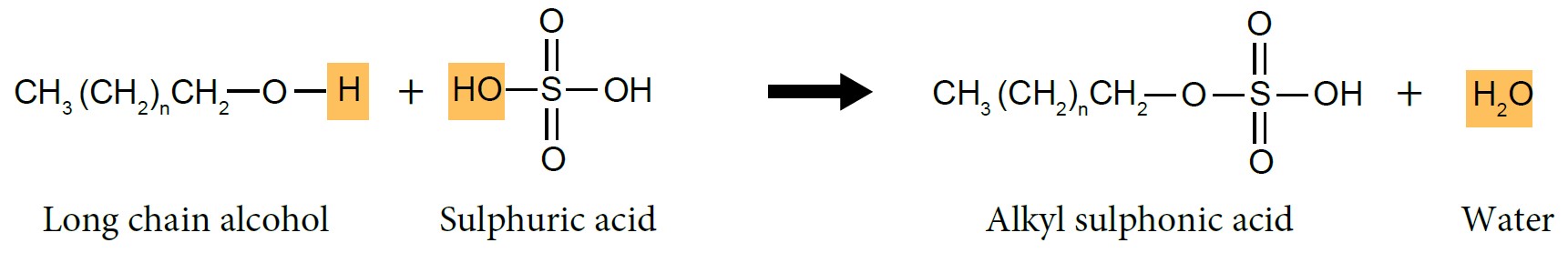
|
- Alkyl sulphonic acid will be neutralised by sodium hydroxide, NaOH solution to produce sodium alkyl sulphate, which is detergent.
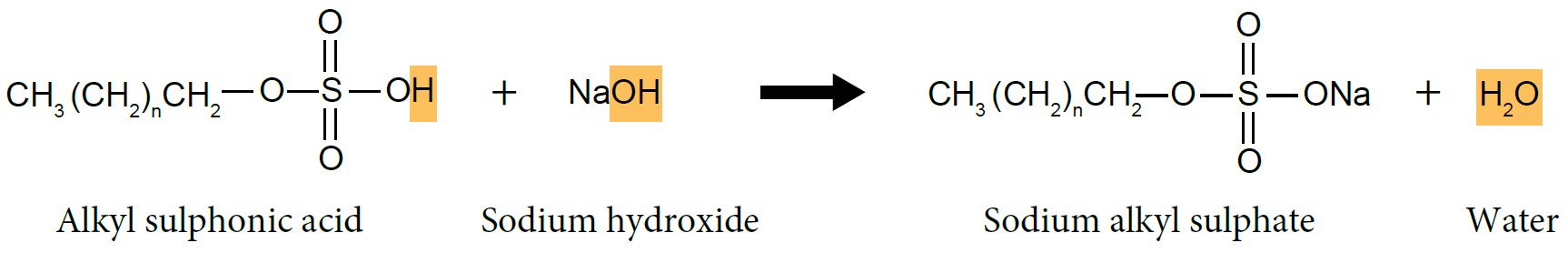
|
|
| |
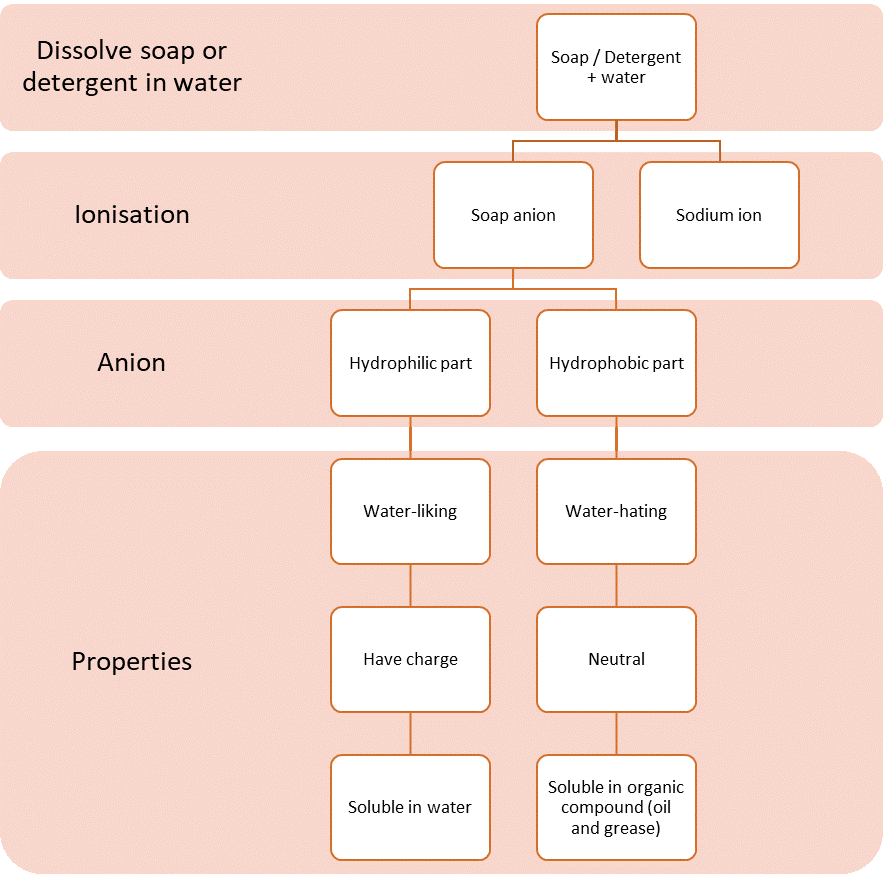
| Cleansing Action of Soap and Detergent |
- Basically, the cleansing action of soap and detergent is the same.
- Soaps and detergents act as emulsifying agents because soap and detergent molecules are soluble in oil or grease and water.
- When soap or detergent is dissolved in water, soap or detergent molecules dissolve to form:
- sodium ion, \(Na^+\) or potassium ion, \(K^+\).
- soap anion or detergent anion.
| Anion and Detergent Anion |
| Soap \(\xrightarrow[]{Water}\) Soap anion + Sodium ion |
| Detergent \(\xrightarrow[]{Water}\) Detergent anion |
| Structural Formula for Soap Anion and Detergent Anion |
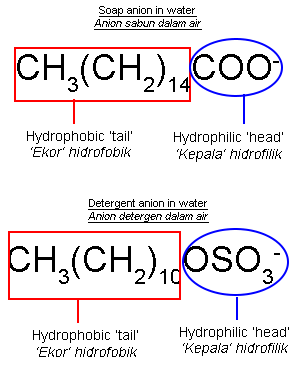 |
- The structures of soap anion and detergent anion consist of two parts, namely:
- hydrophilic part that is soluble in water.
- hydrophobic part that is soluble in oil or grease.
- Both of these properties make soap and detergent effective cleaning agents.
| Step |
Explanation |
| 1 |
- Adding soap or detergent into water will reduce the surface tension of water.
- This increases the water’s ability to wet the surface of the cloth.
|
| 2 |
- Soap or detergent will ionise in water to produce free moving soap anions or detergent anions.
|
| 3 |
- The hydrophilic parts of soap anions or detergent anions dissolve in water.
- The hydrophobic parts dissolve in grease.
|
| 4 |
- Movement of water during scrubbing and agitation causes grease to pull away from the surface of the cloth.
|
| 5 |
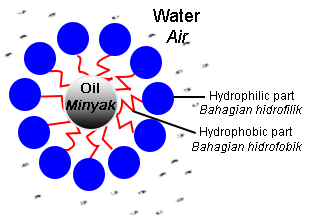
- The hydrophilic parts of soap anions or detergent anions surround the grease.
- Grease floats to the surface of the water.
|
| 6 |
- Grease will break into small droplets.
- The small droplets will not reattach to the surface of the cloth due to the repulsion of negative charges of the hydrophilic parts on the surface of the grease.
- The droplets are suspended in water, forming an emulsion.
- Rinsing with water causes the surface of the cloth to become clean because the grease droplets are left in the water.
|
|
|
| |
| Cleansing Action of Soap and Detergent |
 |
|
| |
| Grease Broken into Droplets of Emulsion |
 |
|
| |
| Comparison of Cleansing Action of Soap and Detergent |
- Water containing calcium ions, \(Ca^{2+}\) and magnesium ions, \(Mg^{2+}\) is called hard water.
- Soap anions combine with the cations to form insoluble salts called scum.
- The formation of scum causes wastage of soap because more soap will be needed for the cleansing action.
- Detergent anions also combine with the cations to form soluble salts in water.
- Therefore, the effectiveness of the detergent’s cleansing action is not affected by hard water.
- The comparison of cleaning action of soap and detergent is as shown below:
| Aspect |
Soap |
Detergent |
| Effectiveness in soft water |
Effective. |
Effective. |
| Effectiveness in hard water |
Less effective |
More effective. |
| Effectiveness in acidic water |
Not effective due to the formation of insoluble organic acid. |
Effective because the organic acid formed is soluble. |
|
|
| |
 |
| |
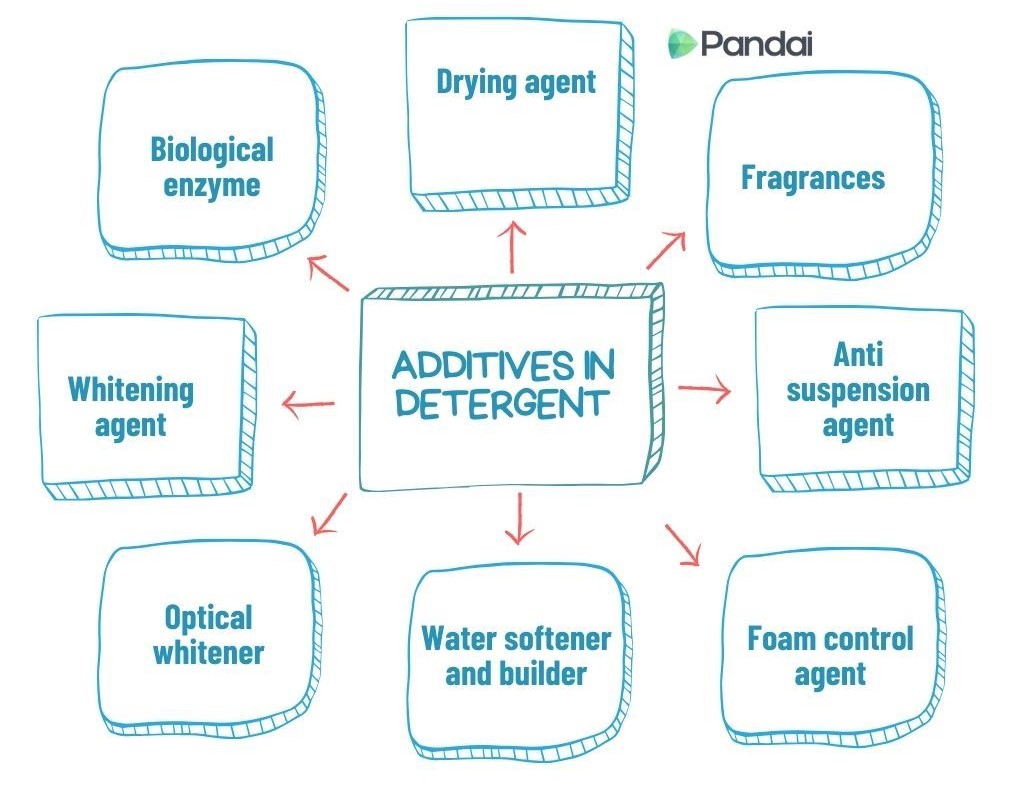
| Additives in Detergent |
| Additives |
Function |
Example |
| Biological enzyme |
To remove protein stains, such as blood, milk and sugar. |
Amylase, protease, cellulase and lipase. |
| Whitening agent |
To change dirt to colourless substance. |
Sodium perborate and Sodium hypochlorite. |
| Optical whitener |
To make clothes become whiter and brighter. |
Fluorescent dyes. |
| Water softener and builder |
To enhance the effectiveness of the detergent by softening the water. |
Sodium tripolyphosphate. |
| Foam control agent |
To control the foam formed by the detergent. |
Alkyl monoethanolamide. |
| Anti suspension agent |
To prevent the removed dirt from redepositing to the clothes. |
Sodium carboxylmethyl-cellulose. |
| Fragrances |
To enhance the fragrance of the detergent and fabric. |
Jasmine and lavender. |
| Drying agent |
To ensure that the detergent powder is always dry in its container. |
Sodium sulphate and sodium silicate. |
|
| |