Chapter 1 : Keseimbangan Redoks
What will you learn in this chapter
Seterusnya, kita akan mengkaji potensi elektrod piawai. Kita akan mempelajari maksudnya dan bagaimana untuk menentukan agen pengoksidaan dan penurunan berdasarkan nilai potensi elektrod piawai mereka.
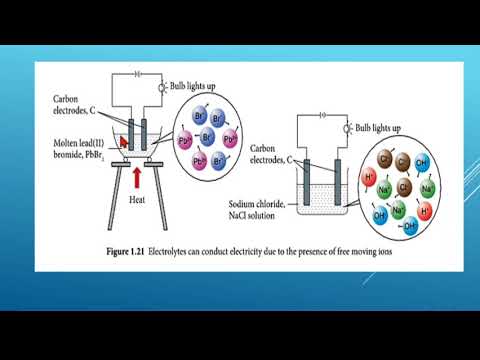
Seterusnya, kita akan meneroka sel volta dan memahami cara tindak balas redoks berlaku dalam sel ini melalui eksperimen. Kita juga akan belajar tentang sel elektrolitik dan elektrolisis. Melalui aktiviti, kita akan memahami bagaimana elektrolisis berfungsi dalam sebatian lebur.
Menjelang akhir bab ini, kita akan mempunyai pemahaman yang baik tentang keseimbangan redoks dan dapat menggunakan pengetahuan kita untuk menyelesaikan masalah dan menganalisis tindak balas redoks.
Live Tuition Recordings

Tutor: Sir Aiman

Tutor: Sir Aiman

Tutor: Sir Aiman

Tutor: Sir Aiman

Tutor: Sir Aiman

Tutor: Sir Aiman

Tutor: Sir Mas Faiz
Experiments
Videos
Practices for this chapter
-
Flashcard
- Keupayaan Elektrod Piawai (1)
- Keupayaan Elektrod Piawai (2)
- Keupayaan Elektrod Piawai (3)
- Keupayaan Elektrod Piawai (4)
- Keupayaan Elektrod Piawai (5)
- Keupayaan Elektrod Piawai (6)
- Pengaratan (1)
- Pengaratan (2)
- Pengaratan (3)
- Pengaratan (4)
- Pengaratan (5)
- Pengaratan (6)
- Pengekstrakan Logam daripada Bijihnya (1)
- Pengekstrakan Logam daripada Bijihnya (2)
- Pengekstrakan Logam daripada Bijihnya (3)
- Pengekstrakan Logam daripada Bijihnya (4)
- Pengekstrakan Logam daripada Bijihnya (5)
- Pengekstrakan Logam daripada Bijihnya (6)
- Pengoksidaan dan Penurunan (1)
- Pengoksidaan dan Penurunan (2)
- Pengoksidaan dan Penurunan (3)
- Pengoksidaan dan Penurunan (4)
- Pengoksidaan dan Penurunan (5)
- Pengoksidaan dan Penurunan (6)
- Sel Elektrolisis (1)
- Sel Elektrolisis (2)
- Sel Elektrolisis (3)
- Sel Elektrolisis (4)
- Sel Elektrolisis (5)
- Sel Elektrolisis (6)
- Sel Kimia (1)
- Sel Kimia (2)
- Sel Kimia (3)
- Sel Kimia (4)
- Sel Kimia (5)
- Sel Kimia (6)
-
Topical Test
- 1.1 Pengoksidaan dan Penurunan - Set 1
- 1.1 Pengoksidaan dan Penurunan - Set 2
- 1.1 Pengoksidaan dan Penurunan - Set 3
- 1.1 Pengoksidaan dan Penurunan - Set 4
- 1.1 Pengoksidaan dan Penurunan - Set 5
- 1.2 Keupayaan elektrod piawai - Set 1
- 1.3 Sel Kimia - Set 1
- 1.3 Sel Kimia - Set 2
- 1.3 Sel Kimia - Set 3
- 1.3 Sel Kimia - Set 4
- 1.4 Sel Elektrolitik - Set 1
- 1.4 Sel Elektrolisis - Set 2
- 1.4 Sel Elektrolitik - Set 3
- 1.4 Sel Elektrolisis - Set 4
- 1.4 Sel Elektrolitik - Set 5
- 1.6 Pengaratan - Set 1
- 1.6 Pengaratan - Set 2
- 1.6 Pengaratan - Set 3
- 1.6 Pengaratan - Set 4
- 1.6 Pengaratan - Set 5